pH in freshwater
In freshwater systems, pH plays an important role in determining the conditions for life.
What is pH?
pH stands for “potential for Hydrogen”. It is the measure of the acidity or alkalinity of water soluble substances.
Why is pH important in freshwater?
pH sets up the conditions for how easy it is for nutrients to be available and how easily things like heavy metals (toxicity for aquatic life) can dissolve in the water. Rivers and lakes generally range between 5 (acidic) and 9 (basic) on the pH scale. Whereas ocean water averages closer to 8.2 (slightly basic). Low pH can reduce how many fish eggs hatch and can make life difficult for fish and macroinvertebrates (the backbone of our water ecosystems).
What does a pH measurement mean?
The most important thing is to first establish a baseline for testing. Based on that number we can determine if something is influencing the water’s health. For example if the pH falls below 5 or over 10 (this can be caused by algae blooms) you will start to see fish life spans and reproduction affected.
Choosing the right pH strip
Discover how we chose our teststrips.
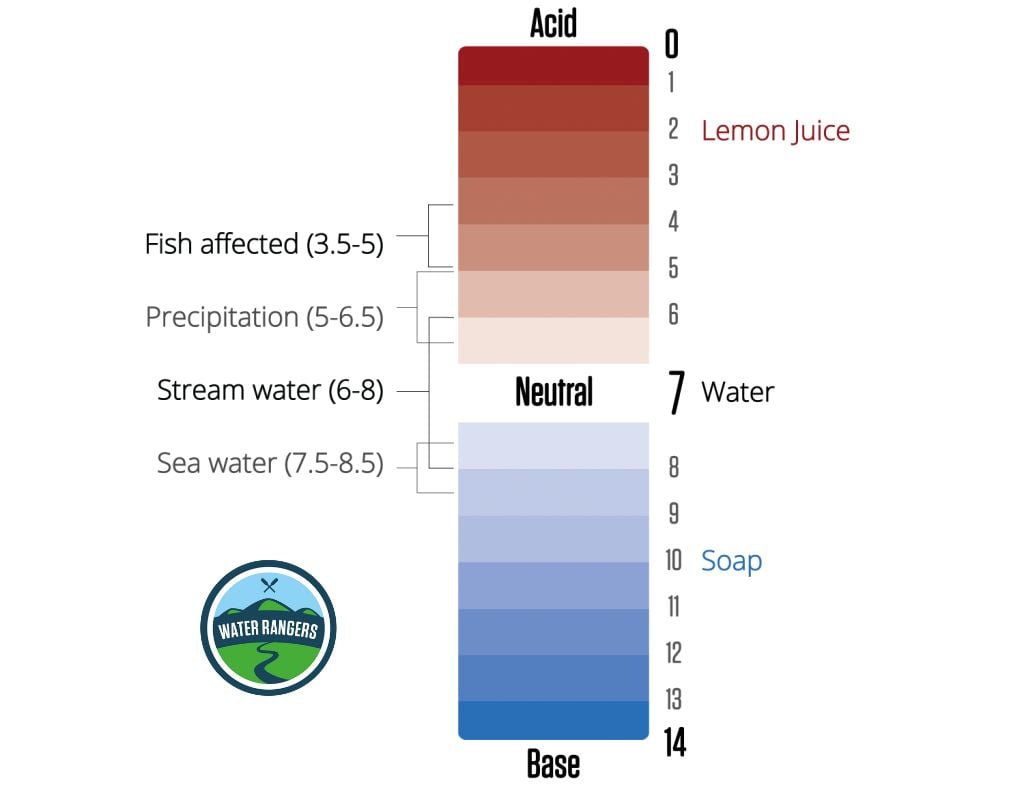
Common values for pH in freshwater
Did you know?
- Coloured water tends to be more acidic, so it may have a lower pH than clean water.
- Like other parameters, you’ll need to create a baseline for what’s a normal pH level (e.g. Ottawa River’s pH is usually around 7, while the Rideau River has a normal pH level around 8).
- pH is important because it sets up conditions for how easy it is for nutrients to be available (which can lead to algae blooms) and how easily things like heavy metals can dissolve in the water (creating toxicity for aquatic life).
- Human influence through dumping can change pH levels. Acid rain, mining run-off, and pine forests all lower the pH of
water systems. - Lakes and rivers have an optimum pH level (average 7.4)
- Fish and organisms can’t survive above or below certain values
- Changes in pH could indicate that an area is in trouble
- Plants affect pH over a day through photosynthesis and respiration: pH will be highest in the afternoon and lowest before sunrise.
- Low pH can reduce how many fish eggs successfully hatch, and make life difficult for fish and macroinvertebrates (the small creatures that are the backbone of our water ecosystems). Amphibians are very sensitive to low pH (their skin is sensitive).
Algae blooms
Harmful algae is a growing concern for lakes and are made worse by excess nutrients from human activity. Algae blooms have an optimum pH between 8.2 – 8.7 and usually appear in late summer/early fall. When algae grows, pH levels go up, and can reach over 10 (all fish die above 10)! Some algae is normal. However, if you you see a lot, be careful as some are toxic!
Water Rangers Protocol
We use test strips for pH. We’ve compared 15 types of test strips, and those made by Taylor have been shown to be the most accurate.
For those looking for more accuracy, inexpensive pen-style meters have proven to be very accurate. Water Rangers offers this pH meter.
How to test for pH in freshwater with Taylor test strips
- Rinse sample cup 3 times.
- IMPORTANT! Make sure your hands are dry, since moisture in the container will ruin strips.
- Shake out 1 test strip, close bottle.
- Dip the entire strip in the water. Remove after 2 seconds.
- Wait 20 seconds before reading.
- Compare colours with guide on side of bottle. Line up the colour strips vertically so that you can compare along the spectrum for each value.

How to calibrate and use the pH meter
Calibration process
The pH meter needs to be calibrated often because we need to ensure our pH meters are accurate at all times. Without the calibrations, the meter readings can drift over time, leading to inaccuracies.
If you are testing on a daily basis, the meters should be calibrated at least once a day.
Items you will need:
- pH packets provided in the your testkits with the pH of 4, 7, 10.
- Jars or bottles that can hold at least 250 mL of liquid.
- 4 Liters of distilled water for calibration solutions and rinsing.
- Water Rangers cloth for cleaning/wiping.
To perform the calibration you will need to follow these steps:
- Fill 3 clean, resealable jars or bottles with 250mL of distilled water each
- Label containers for each pH solution: 4, 7, 10.
- Open calibration packets and pour contents of each pH range into separate containers (follow the labels).
- Stir with clean utensil and wait for 30 minutes or until the powders have completely dissolved.
- Take out your pH meter and power it on.
- Press “CAL” on the pH meter to start the calibration process. The meter will flash 4, 7, and 10 in a cycle. This means it is ready to be calibrated.
- Always start with the solution with the pH of 7, and then move onto 4 or 10 in no particular order for better accuracy.
- Dip the meter into the solution, and wait. The meter will recognize the solution’s pH and indicate it on the screen. For the first calibration, 7!
- Once the meter reads a stable value, without pressing a single key, the meter will automatically save the value and return to the measurement screen.
- Rinse and repeat this process for pH 4 and pH 10. Make sure to rinse meter every time you do a calibration.
- Store calibration solution in a cool location. They can last up to 3-4 weeks and can be reused.
Taking a measurement
- Rinse sample cup 3 times before taking the final sample for the measurement. If possible and safe to do so, you can also dip the meter right into the waterbody.
- Dip the meter into the water and wait for the number on the screen to stabilize before recording the number. You can even press the “HLD” or the hold button to freeze the reading so you can take notes without it changing.
- Since pH is affected by water temperature, you should also use this to record water temperature.
- After the measurement, be sure to rinse the probe with distilled water before the next measurement or before putting it away.
Things to keep in mind!
- To prolong the life of the meter, make sure to keep the pH glass bulb wet by using the cap to protect and store the electrode.
- Make sure to always rinse the probe of the meter before using and between measurements.
- NEVER touch the glass bulb as it is very delicate and can alter your readings.
- If you are testing on a monthly basis, you will have to calibrate the meter every time before heading out.
Start testing for pH using these products:
-
Product on sale
 pH meterOriginal price was: $60.00.$55.00Current price is: $55.00.
pH meterOriginal price was: $60.00.$55.00Current price is: $55.00. -
 Compact Freshwater Testkit$140.00
Compact Freshwater Testkit$140.00 -
 Teststrips$16.00
Teststrips$16.00
Contributing to the community!
Water Rangers is citizen-scientist led. So, if you have any questions, ideas, or notice any errors related to our tests, please tell us!
